Britain approves Merck's COVID-19 pill in world first
Author of the article:Reuters
Reuters
Publishing date:Nov 04, 2021 • 21 hours ago • 4 minute read • Join the conversation
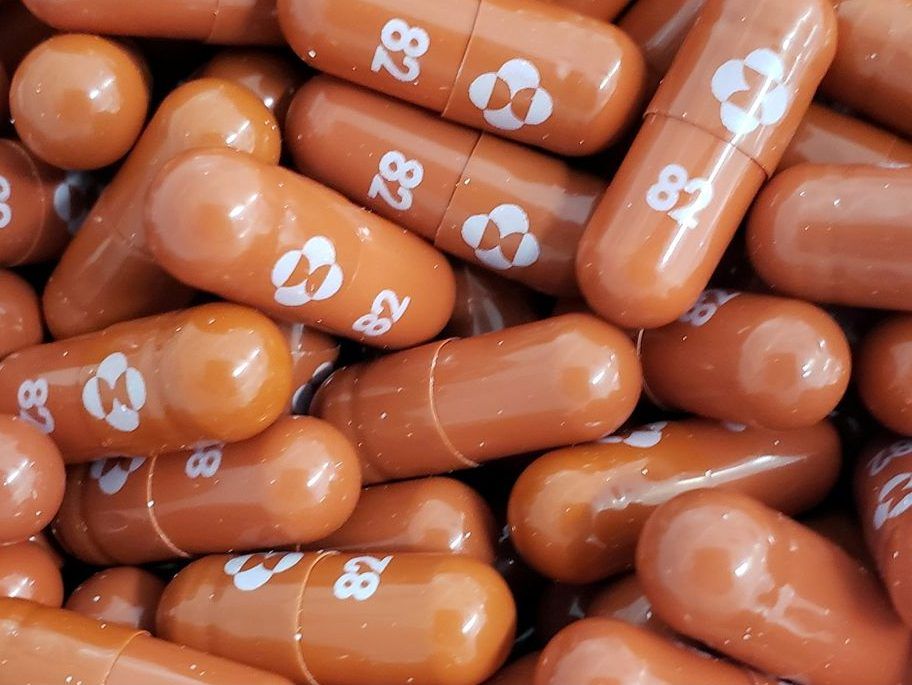
An experimental COVID-19 treatment pill called molnupiravir being developed by Merck & Co Inc and Ridgeback Biotherapeutics LP, is seen in this undated handout photo released by Merck & Co Inc and obtained by Reuters May 17, 2021.
An experimental COVID-19 treatment pill called molnupiravir being developed by Merck & Co Inc and Ridgeback Biotherapeutics LP, is seen in this undated handout photo released by Merck & Co Inc and obtained by Reuters May 17, 2021. PHOTO BY MERCK & CO INC /via REUTERS
Article content
Britain on Thursday became the first country in the world to approve a potentially game-changing COVID-19 antiviral pill jointly developed by U.S.-based Merck and Ridgeback Biotherapeutics, in a boost to the fight against the pandemic.
Advertisement
STORY CONTINUES BELOW
Article content
Britain’s Medicines and Healthcare products Regulatory Agency (MHRA) recommended the drug, molnupiravir, for use in people with mild to moderate COVID-19 and at least one risk factor for developing severe illness, such as obesity, older age diabetes, and heart disease.
It will be administered as soon as possible following a positive COVID-19 test and within five days of the onset of symptoms, the regulator said, citing clinical data.
The green light is the first for an oral antiviral treatment for COVID-19 and the first for a COVID-19 drug that will be administered widely in the community. U.S. advisers will meet this month to vote on whether molnupiravir should be authorized.
Treatments to tackle the pandemic, which has killed more than 5.2 million people worldwide, have so far focused mainly on vaccines. Other options, including Gilead’s infused antiviral remdesivir and generic steroid dexamethasone, are generally only given after a patient has been hospitalized.
Advertisement
STORY CONTINUES BELOW
Article content
Merck’s Molnupiravir has been closely watched since data last month showed it could halve the chances of dying or being hospitalized for those most at risk of developing severe COVID-19 when given early in the illness.
Molnupiravir, which will be branded as Lagevrio in Britain, is designed to introduce errors into the genetic code of the coronavirus that causes COVID-19 and is taken twice a day for five days.
Professor Stephen Powis, national medical director for the National Health Service (NHS) in England, said the drug would be administered to patients at higher risk of complications as Britain heads into one of the most challenging winters ever.
A wider rollout will follow if it is clinically and cost effective in reducing hospitalizations and death, he added.
Advertisement
STORY CONTINUES BELOW
Article content
“We are now working across government and the NHS to urgently get this treatment to patients initially through a national study so we can collect more data on how antivirals work in a mostly vaccinated population,” UK vaccines minister Maggie Throup told parliament.
PRESSURES
The speedy approval in Britain, which was also the first Western country to approve a COVID-19 vaccine, comes as it struggles to tame soaring infections.
Britain has about 40,000 daily cases of COVID-19, according to the latest seven-day average. That is second only to the roughly 74,000 a day in the United States, which has five times more people, and has fueled criticism of the government’s decision to abandon most pandemic-related restrictions
Advertisement
STORY CONTINUES BELOW
Article content
Data released on Wednesday night showed COVID-19 prevalence in England hit its highest level on record last month, led by a high numbers of cases in children and a surge in the south-west of the country.
Pressure is growing on the government to implement its “Plan B” aimed at protecting the NHS from unsustainable demands, involving mask mandates, vaccine passes and work-from-home orders.
Many other big economies, including Germany, France and Israel, have either retained some basic COVID-19 measures like mask mandates or reintroduced them in response to rising cases.
The UK government has said its focus remains on administering vaccine boosters and inoculating 12 to 15-year-olds.
“With no compromises on quality, safety and effectiveness, the public can trust that the MHRA has conducted a robust and thorough assessment of the data (on molnupiravir),” MHRA chief June Raine said in a statement.
Advertisement
STORY CONTINUES BELOW
Article content
Last month, Britain agreed a deal with Merck to secure 480,000 courses of molnupiravir.
Professor Penny Ward, an independent pharmaceutical physician, welcomed the approval, but said the NHS needed to outline its plans for rollout and cautioned that supplies were likely to be tight given the strong global demand.
“Comments made by Mr Javid today suggest that it may be made available via a clinical trial, presumably to investigate its effectiveness in vaccinated patients with breakthrough infections, as the original study incorporated unvaccinated adults,” she said.
If given to everyone becoming unwell, the nearly half a million courses would not last very long given the more than 40,000 current daily case rate, she said.
Advertisement
STORY CONTINUES BELOW
Article content
TREATMENT RACE
In a separate statement, Merck said it expected to produce 10 million courses of the treatment by the end of this year, with at least 20 million set to be manufactured in 2022.
The U.S. based drugmaker’s shares were up 2.1% at $90.54 before the market open.
Pfizer and Roche are also racing to develop easy-to-administer antiviral pills for COVID-19. Pfizer last month began a large study of its oral antiviral drug for the prevention of COVID-19 in people exposed to the coronavirus.
Merck’s molnupiravir is also being studied in a late-stage trial for preventing infection.
Viral sequencing done so far has shown molnupiravir is effective against all variants of the coronavirus, Merck has said, including the more-infectious Delta, which is responsible for the worldwide surge in hospitalizations and deaths recently.
While it is not yet clear when Merck will deliver doses to Britain, the company has said it is committed to providing timely access to its drug globally with plans for tiered pricing aligned with a country’s ability to pay.
Merck is also in talks with generic drugmakers about expanding manufacturing licenses to build supply of the treatment.
Antibody cocktails like those from Regeneron and Eli Lilly have also been approved for non-hospitalized COVID-19 patients, but have to be given intravenously.
The antiviral was found to be safe and effective following a stringent review of the available evidence.

gov.uk
Britain on Thursday became the first country in the world to approve a potentially game-changing COVID-19 antiviral pill jointly developed by U.S.-based Merck and R…

torontosun.com